-
Jae Kyoung Kim
Despite dramatic advances in experimental techniques, many facets of intracellular dynamics remain hidden, or can be measured only indirectly. In this talk, I will describe three strategies to analyze timeseries data from biological systems with hidden parts: replacement of hidden components with either time delay, quasi-steady-state or random regulatory process. Then, I will illustrate how the simplification with the time delay can be used to understand the processes of protein synthesis, which involves multiple steps such as transcription, translation, folding and maturation, but typically whose intermediates proteins cannot be measured. Furthermore, I will illustrate how the simplification with the quasi-steady-state can be used to develop an accurate method to estimate drug clearance, which occurs in multiple steps of metabolism, which greatly improved the canonical approach used in more than 65,000 published papers for last 30 years. Finally, I will describe a systematic modeling selection approach to identify hidden regulatory biochemical connections leading to the observed timeseries data. Then, I will illustrate how we applied the approach to find the connection between the circadian clock and cell cycle checkpoints.
-

Jae Kyoung Kim
The development of luciferase markers and other experiment techniques has allowed
measurement of the timecourses of the expression of genes and proteins with remarkable
accuracy. Since this data has been used to construct many mathematical models, it is important
to ask if this problem of model building is well-posed. Here, we focus on a common form of
ordinary differential equation (ODE) models for biological clocks, which consist of production
and degradation terms, and assume we have an accurate measurement of their solution. Given
these solutions, do ODE models exist? If they exist, are they unique? We show that timecourse
data can sometimes, but not always determine the unique quantitative relationships (i.e.
biochemical rates) of network species. In other cases, our techniques can rule out functional
relationships between network components and show how timecourses can reveal the underlying
network structure. We also show that another class of models is guaranteed to have existence and
uniqueness, although its biological application is less clear. Our work shows how the
mathematical analysis of the process of model building is an important part of the study of
mathematical models of biological clocks
-
Jae Kyoung Kim
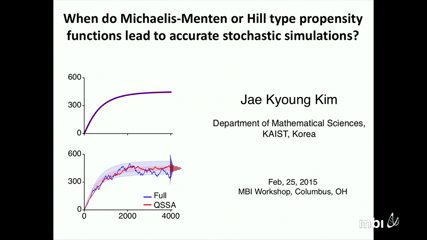
The non-elementary reaction functions (e.g. Michaelis-Menten or Hill functions) are used to reduce the determinsitic models of biochemical networks. Such deterministic reductions are frequently a basis for heuristic stochastic models in which non-elementary reaction functions are used as propensities of Gillespie algorithm. Despite the popularity of this heuristic stochastic simualtions, it remains unclear when such stochastic reductions are valid. In this talk, I will present conditions under which the stochastic models with the non-elementary propensity functions accurately approximate the full stochastic models. If the validity condition is satisfied, we can perform accurate and computationally inexpensive stochastic simulation without converting the non-elementary functions to the elementary functions (e.g. mass action kinetics).
 Jae Kyoung KimDespite dramatic advances in experimental techniques, many facets of intracellular dynamics remain hidden, or can be measured only indirectly. In this talk, I will describe three strategies to analyze timeseries data from biological systems with hidden parts: replacement of hidden components with either time delay, quasi-steady-state or random regulatory process. Then, I will illustrate how the simplification with the time delay can be used to understand the processes of protein synthesis, which involves multiple steps such as transcription, translation, folding and maturation, but typically whose intermediates proteins cannot be measured. Furthermore, I will illustrate how the simplification with the quasi-steady-state can be used to develop an accurate method to estimate drug clearance, which occurs in multiple steps of metabolism, which greatly improved the canonical approach used in more than 65,000 published papers for last 30 years. Finally, I will describe a systematic modeling selection approach to identify hidden regulatory biochemical connections leading to the observed timeseries data. Then, I will illustrate how we applied the approach to find the connection between the circadian clock and cell cycle checkpoints.
Jae Kyoung KimDespite dramatic advances in experimental techniques, many facets of intracellular dynamics remain hidden, or can be measured only indirectly. In this talk, I will describe three strategies to analyze timeseries data from biological systems with hidden parts: replacement of hidden components with either time delay, quasi-steady-state or random regulatory process. Then, I will illustrate how the simplification with the time delay can be used to understand the processes of protein synthesis, which involves multiple steps such as transcription, translation, folding and maturation, but typically whose intermediates proteins cannot be measured. Furthermore, I will illustrate how the simplification with the quasi-steady-state can be used to develop an accurate method to estimate drug clearance, which occurs in multiple steps of metabolism, which greatly improved the canonical approach used in more than 65,000 published papers for last 30 years. Finally, I will describe a systematic modeling selection approach to identify hidden regulatory biochemical connections leading to the observed timeseries data. Then, I will illustrate how we applied the approach to find the connection between the circadian clock and cell cycle checkpoints. Jae Kyoung KimThe development of luciferase markers and other experiment techniques has allowed
Jae Kyoung KimThe development of luciferase markers and other experiment techniques has allowed Jae Kyoung KimThe non-elementary reaction functions (e.g. Michaelis-Menten or Hill functions) are used to reduce the determinsitic models of biochemical networks. Such deterministic reductions are frequently a basis for heuristic stochastic models in which non-elementary reaction functions are used as propensities of Gillespie algorithm. Despite the popularity of this heuristic stochastic simualtions, it remains unclear when such stochastic reductions are valid. In this talk, I will present conditions under which the stochastic models with the non-elementary propensity functions accurately approximate the full stochastic models. If the validity condition is satisfied, we can perform accurate and computationally inexpensive stochastic simulation without converting the non-elementary functions to the elementary functions (e.g. mass action kinetics).
Jae Kyoung KimThe non-elementary reaction functions (e.g. Michaelis-Menten or Hill functions) are used to reduce the determinsitic models of biochemical networks. Such deterministic reductions are frequently a basis for heuristic stochastic models in which non-elementary reaction functions are used as propensities of Gillespie algorithm. Despite the popularity of this heuristic stochastic simualtions, it remains unclear when such stochastic reductions are valid. In this talk, I will present conditions under which the stochastic models with the non-elementary propensity functions accurately approximate the full stochastic models. If the validity condition is satisfied, we can perform accurate and computationally inexpensive stochastic simulation without converting the non-elementary functions to the elementary functions (e.g. mass action kinetics).